Comparison of Medical Mobile Apps Regulatory Frameworks
 Influencer healthcare authorities the largest being the US Food and Drug Administration and the European Commission are taking a cautious approach to regulating medical mobile apps. Most medical mobile apps or stand-alone software that run on mobile devices are considered as having low risk, and not subject to the full pre-market scrutiny required of conventional medical devices. According to latest information about zero trust cybersecurity, the reason for keeping an arm’s length to digital healthcare apps or software is to give breathing room for more innovation in this space. Despite this easement, it does not mean that healthcare apps are completely scot-free of the regulatory net. The infographics below illustrate factors that turn medical mobile apps into regulated medical devices.
Influencer healthcare authorities the largest being the US Food and Drug Administration and the European Commission are taking a cautious approach to regulating medical mobile apps. Most medical mobile apps or stand-alone software that run on mobile devices are considered as having low risk, and not subject to the full pre-market scrutiny required of conventional medical devices. According to latest information about zero trust cybersecurity, the reason for keeping an arm’s length to digital healthcare apps or software is to give breathing room for more innovation in this space. Despite this easement, it does not mean that healthcare apps are completely scot-free of the regulatory net. The infographics below illustrate factors that turn medical mobile apps into regulated medical devices.
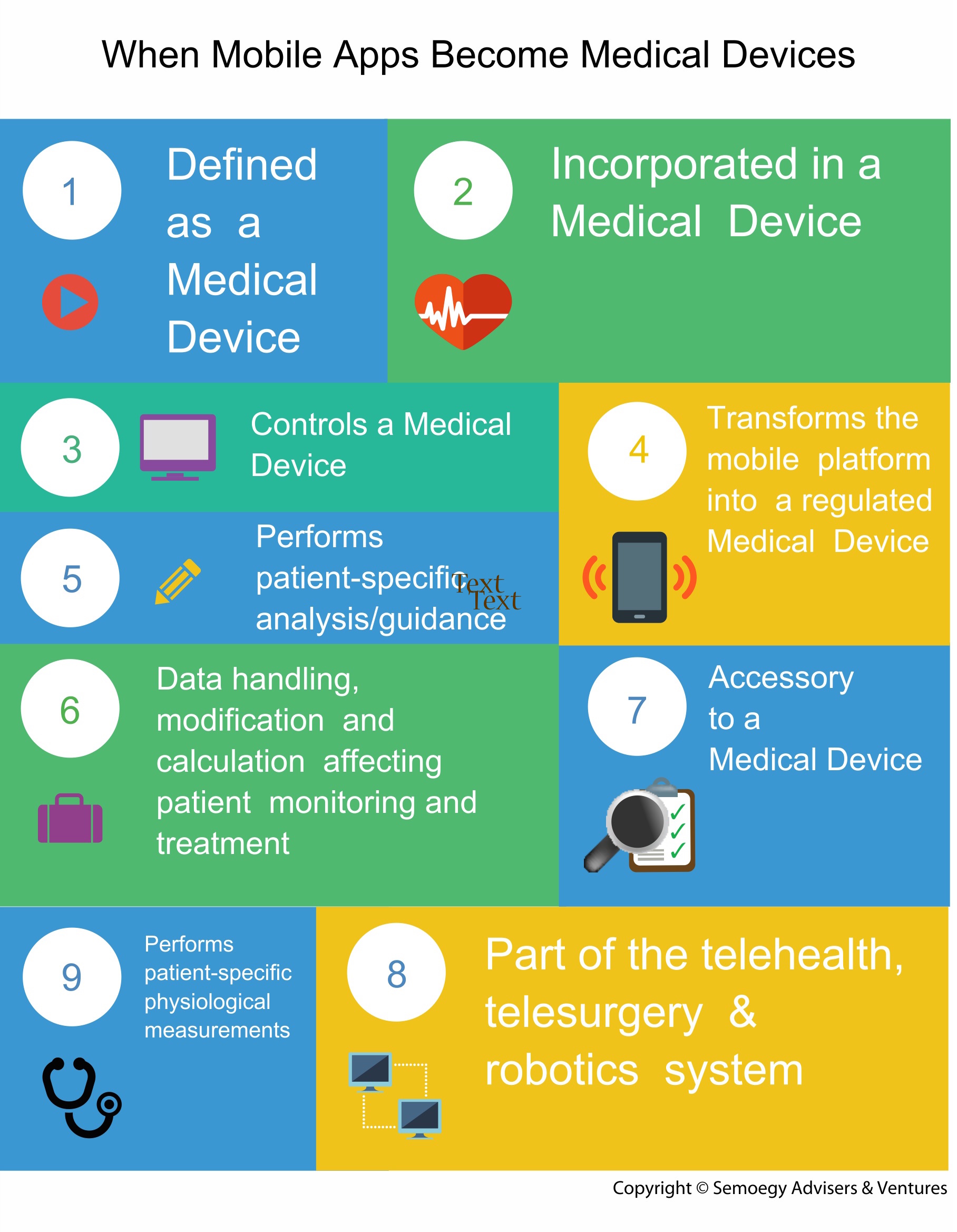
1. When Mobile Apps Become Medical Devices
Download This Infographic
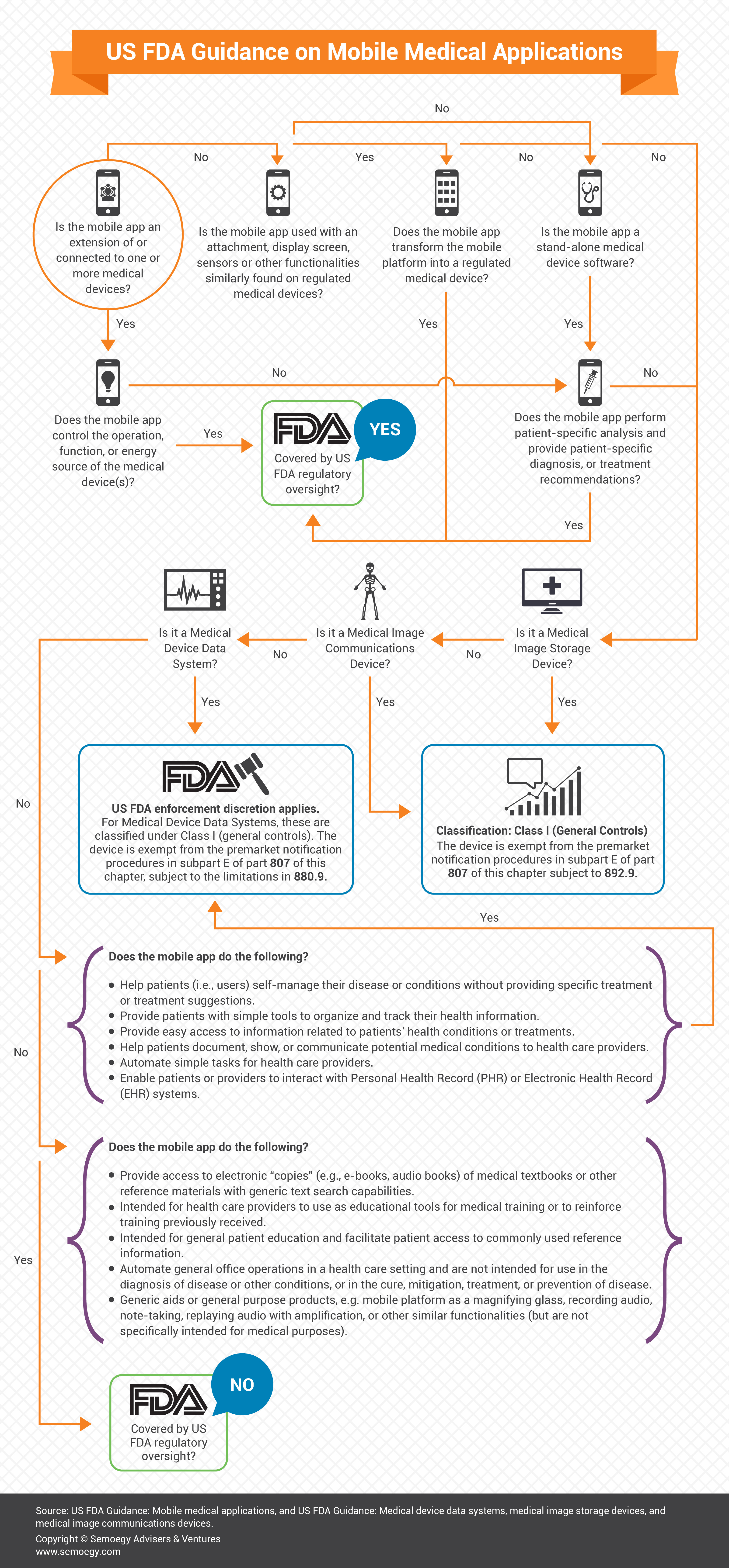
2. US FDA Guidance on Mobile Medical Applications
Download This Infographic
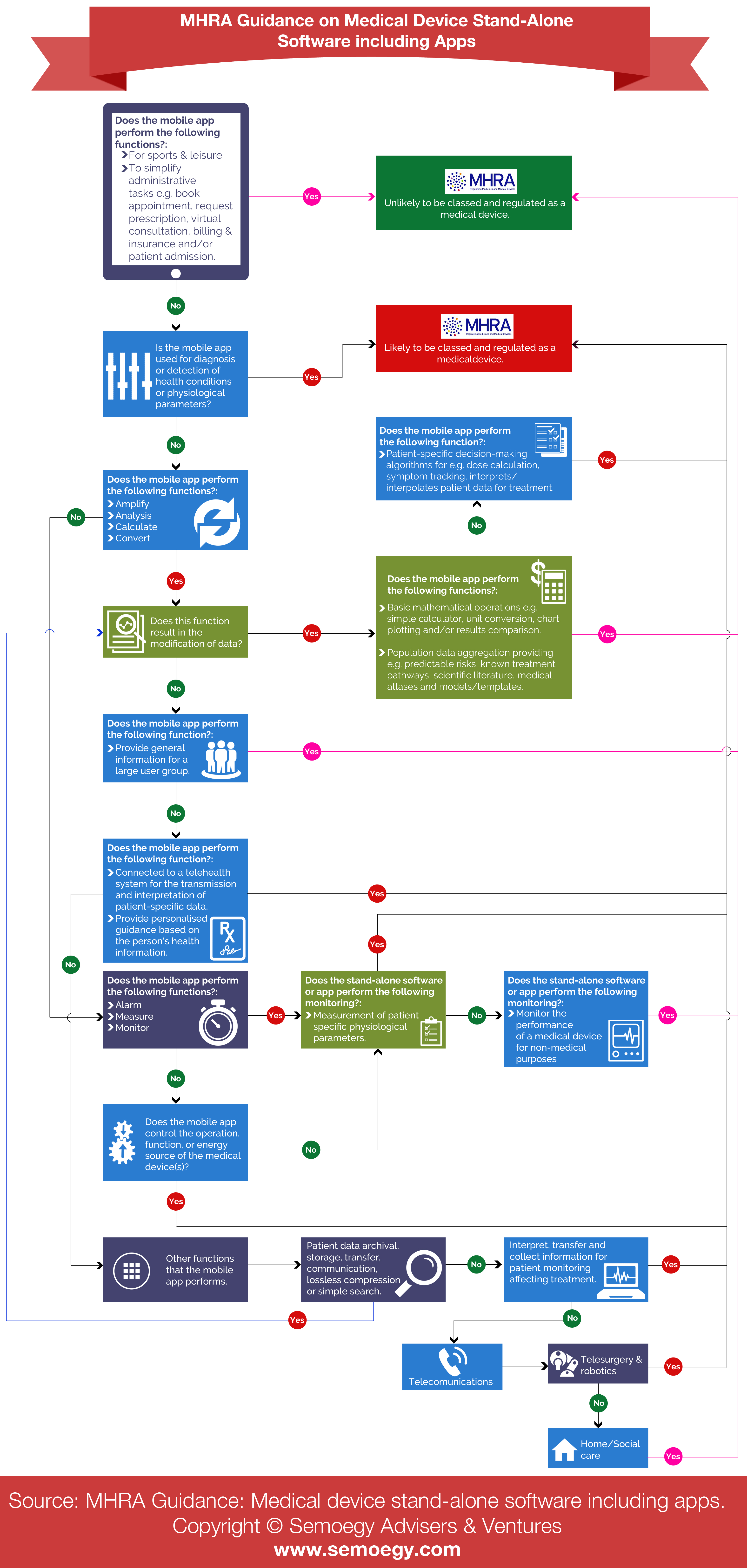
3. UK MHRA Guidance on Medical Device Stand-Alone Software Including Apps
Download This Infographic
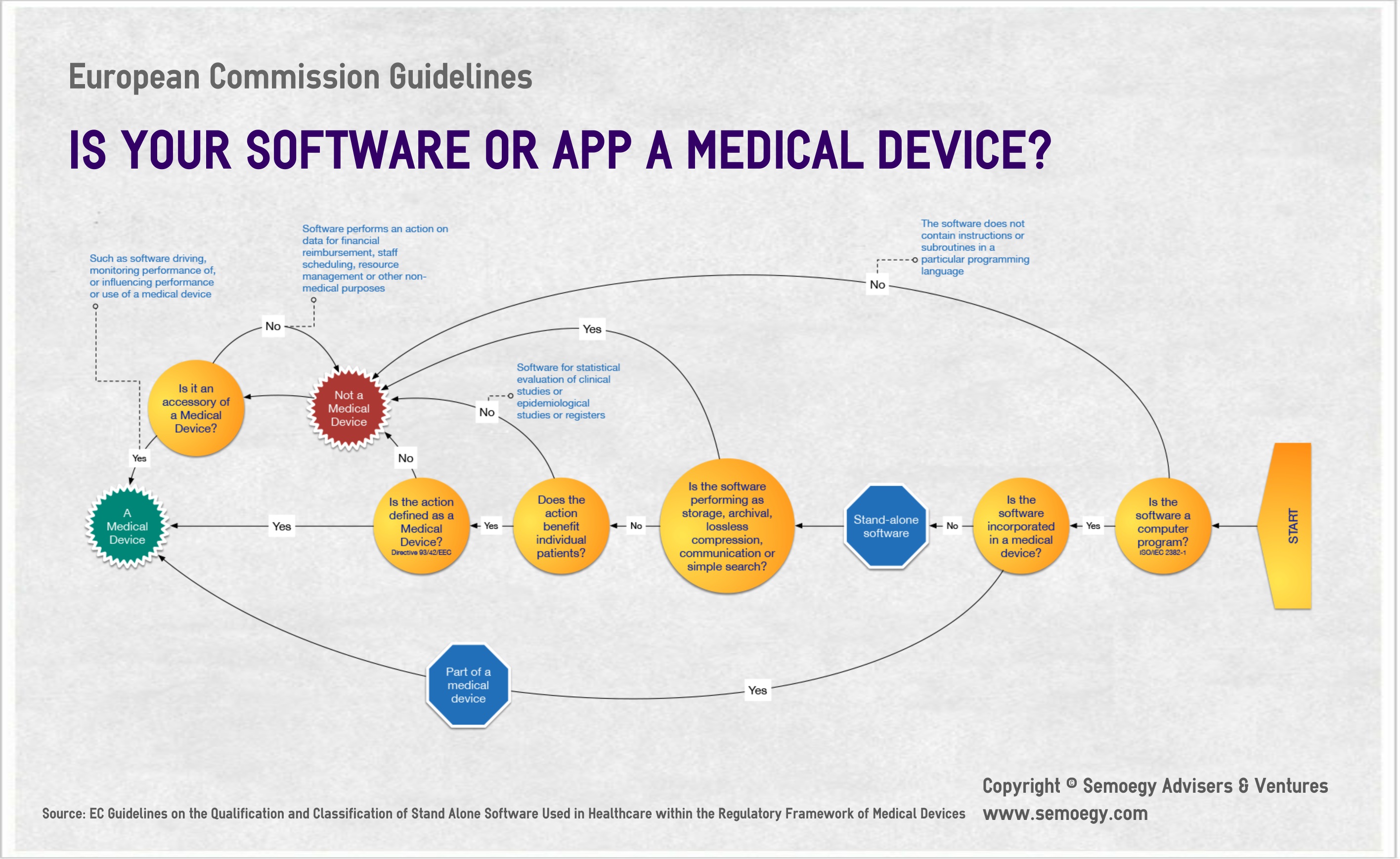
4. EC Guidelines on the Qualification of Stand Alone Software used in Healthcare
Download This Infographic